
The general formula of the amino acid is. All proteins are polymers of amino acids and all these acids except two have an amino group attached to the carbon atom next to the carboxyl group. The amino group (-NH2) is basic while the carboxyl group (-COOH) is acidic in nature. General Structural Chemistry of Amino acids:Īmino acids are a group of organic compounds containing two functional groups – amino and carboxyl. The amino group is basic while the carboxyl group (-COOH) is acidic in nature”. “A substance which has both carboxyl and amino group in the same molecule is called ‘ amino acid’. Chain of amino acid or bio molecules called protein - 3d illustration beta alanine, b-alanine, amino acid, molecular structures, 3d rendering, Glutamate.
Other amino acids are found in certain proteins, but in almost all cases these additional amino acids result from the modification of one of the magic 20 after the protein formed. Francis Crick (who with James Watson determined the structure of DNA) labeled this set of amino acids the magic 20.
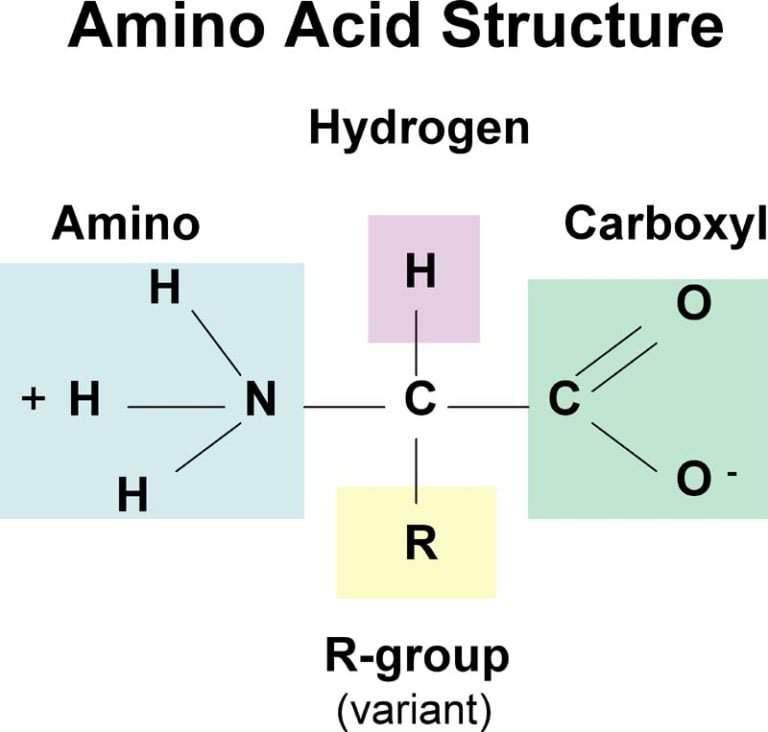
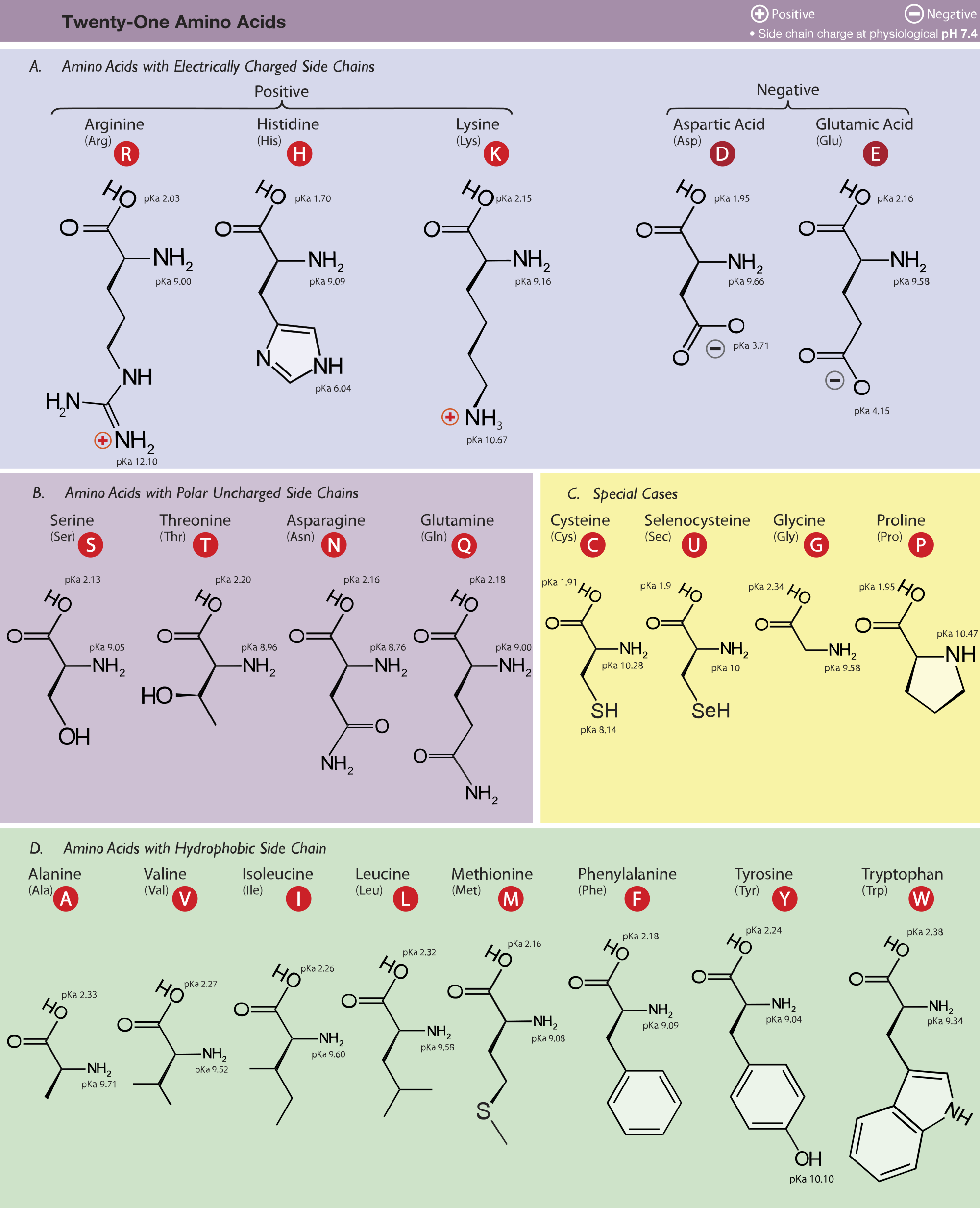
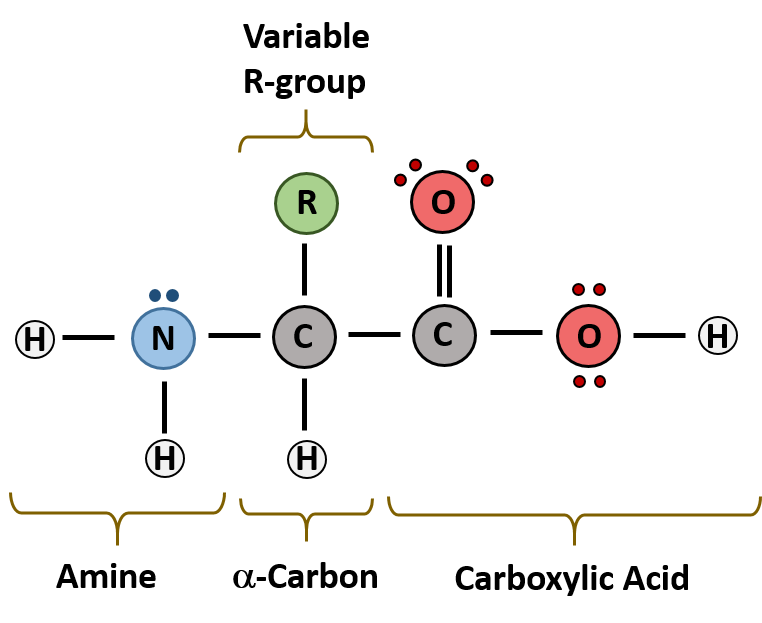
and attached to it are four groups a hydrogen, a carboxylic acid group, an amine group, and an R-group, sometimes referred to as a variable group or side chain. At the center of each amino acid is a carbon called the. There are more than 300 known natural amino acids however, only 20 of them are used in protein synthesis. All amino acids have the same basic structure, shown in Figure 2.1. The biologically important amino acids are the alph-amino acids that have the amine and acid groups attached to the same carbon atom. Amino acids are relatively simple molecules containing both an amine group and an acid group. Download Amino acids Study material FREEīefore going to discuss what is amino acids, we want to discuss few points on the historical points.General Structural Chemistry of Amino acids:.


 0 kommentar(er)
0 kommentar(er)
